In resp. white light and short wave UV
Afghanite: Koksha Valley, Khash & Kuran Wa Munjan Districts, Badakhshan Province, Afghanistan (type locality)
Fluorescence is "vibronic" due to the presence of the disulfide-ion [S2]2- in "cages" in the aluminosilicate structure.
Agrellite with albite: Kipawa alkaline complex, Les Lacs-du-Témiscamingue, Témiscamingue RCM, Abitibi-Témiscamingue, Québec, Canada
The magenta fluorescence of agrellite is very complex. The largest emission happen in the ultraviolet. Various rare earth elements and some divalent manganese contribute to the fluorescence. Albite fluoresces weak red due to the presence of a little trivalent iron, which replaces aluminum.
Agrellite with thorite: Kipawa alkaline complex, Les Lacs-du-Témiscamingue, Témiscamingue RCM, Abitibi-Témiscamingue, Québec, Canada
The green fluorescence of thorite is usually attributed to the uranyl ion. However, Gorobets states that tetravalent titanium replacing silicon causes this fluorescence. The emission peak would then lie around 530 nm, which is indeed in the middle of the green part of the spectrum.
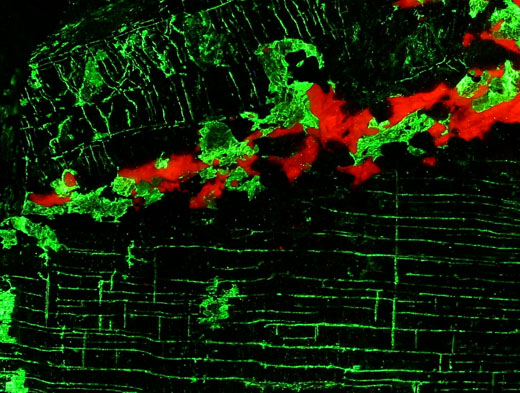
Armstrongite: Khan Bogdo Massive (Khan-Bogdinskii), Gobi Desert, Mongolia (TL)
Named after Neil Armstrong, the first human to set foot on another celestial body..
Benitoite: California State Gem Mine (Dallas Gem Mine), Dallas Gem Mine area, San Benito River headwaters area, New Idria District, Diablo Range, San Benito Co., California, USA
The blue fluorescence is said to be intrinsic. There is also often a red fluorescence seen under longwave UV. That might be caused by a small amount of monovalent copper replacing barium. The latter, however, has not yet been demonstrated.
Beryl, var. goshenite: Pingwu beryl mine (Xuebaoding W-Sn-Be deposit), Huya township, Mt Xuebaoding, Pingwu Co., Mianyang Prefecture, Sichuan Province, China
It seems like this beryl fluoresces firmly blue in short-wave UV. In fact, however, we see the fluorescence of scheelite that is enclosed as very fine dust particles in this beryl crystal.
Beryl, var. emerald: Mun. de Muzo, Vasquez-Yacopí Mining District, Boyacá Department, Colombia
Emerald fluoresces rather seldom under UV. Under violet laser light of 405 nm, however, many emeralds light up bright red. For an explanation: see spectrum.
Catapleiite: Norra Kärr, Gränna, Jönköping, Småland, Sweden
For an explanation: see spectrum.
Dumortierite: Dehesa, San Diego Co., California, USA
For an explanation: see spectrum.
Dumortieriet:
Cabora Bassa Dam, Tete Province, Mozambique
Also here [TiO6]8-, tetravalent titanium, and posibly a little trivalent chromium.
Hardystonite (blue) met willemite (green), esperite (yellow) and calcite (oranje).
Franklin Mine, Franklin, Franklin Mining District, Sussex Co., New Jersey, USA
For an explanation: see spectrum.
Tremolite var. hexagonite: Gouverneur Mine, Fowler, St. Lawrence Co., New York, USA
Fluoresces due to a little manganese replacing calcium and/or magnesium.
In: white light
In long wave UV
In: violet laser 405 nm
Hiortdahlite: Lille Arøya (Midtre Arøy; Øvre Arøy; Övre-Arö), Langesundsfjorden, Larvik, Vestfold, Norway
A little bit of manganese replacing calcium is probably the cause of this fluorescence.
Laumontite on prehnite: Pune District, Maharashtra, India
Medium strong fluorescence, Probably due to prescence of organic material (humic acids)
In resp. white light and long wave UV
In resp. white light and short wave UV
In resp. white light and short wave UV
In resp. white light and short wave UV
In resp. white light and short wave UV
In resp. white light and 405 nm laser light
In resp. white light and short wave UV
In resp. white light and short wave UV
In resp. white light and short wave UV
short wave UV
In resp. white light and short wave UV
In resp. white light and short wave UV
In resp. white light and long wave UV
In mid-range UV
Zircon: Matongo, Burundi
For an explanation: see spectrum.
Willemite and calcite in tephroite, Franklin, Franklin Mining District, Sussex Co., New Jersey, USA
exsolution lamellae of willemite (Zn2SiO4) in tephroite (Mn2SiO4). At high temperatures this was all one mineral, a zincian tephroite, but as temperatures declined and the lattice contracted, the zinc ions could no longer fit in the crystallographic Mn sites of tephroite. Thus the Zn was "expelled" to form sheets of willemite along crystallographic planes within the tephroite. This is a solid-state reaction as temperatures declined during metamorphism. See close-up photo below. (Many thanks to Earl Verbeek for sharing his knowledge and Dick Bostwick for the specimen)
Both the red fluorescence of calcite and the green fluorescence of the willemite are caused by manganese replacing calcium and zinc respectively.
In resp. white light and short wave UV
Stilbite: Nashik District (Nasik District), Maharashtra, India
A nice bowtie-shaped specimen. Stilbite is not especially known for its fluorescent properties but every now and then you come across a specimen that is worth displaying in the UV-cabinet. This one has a strong whiteish fluorescence, due to organic matter that has been included.
In resp. white light and short wave UV
In resp. white light and short wave UV
Esperite,Willemite and calcite, Franklin Mine, Franklin, Franklin Mining District, Sussex Co., New Jersey, USA
all the colors that are visible in the fluorescence are caused by the same activator; manganese. Truly the most versatile activator you'll ever come across.
red = calcite Mn2+ repl. Ca2+
yellow = esperite Mn2+ repl. Ca2+
green = willemite Mn2+ repl. Zn2+
Click on the photo to magnify. Photo in halogen light and 405 nm laser lightt resp.
Chromian-tremolite (tremolite-Cr)
Merelani Hills, Lelatema Mts, Simanjiro District, Manyara Region, Tanzania
A perfectly shaped crystal, which is colored green by the presence of chromium. Apparently, only the upper part of the crystal has absorbed a sufficient amount of the activator to allow it to fluoresce. The green color of the lower part of the specimen is not fluorescence, but rather reflection and transmission of the fierce fluorescence of the putty in which the crystal is set.
Natrolite
Mont Saint-Hilaire, La Vallée-du-Richelieu RCM, Montérégie, Québec, Canada
The medium strong green fluorescence is caused by the inclusion of the uranyl ion.
In resp. white light and SW UV
Spodumene, variety kunzite
Dara-i-Pech pegmatite field, Chapa Dara District, Konar Province, Afghanistan
Top of a long thin crystal (130 x 30 x 5 mm). Due to the presence of manganese, which partly replaces aluminum, the violet color of kunzite arises. Kunzite exhibits pleochroism: The violet color of the manganese centers is much deeper when seen along the c-axis of the crystal. The photograph under halogen light is therefore taken with illumination through the bottom of the crystal, which makes the color much more intense. Click on the prism icon for explanation of fluorescence.
Click on the photos to zoom in
Photos in resp. halogen, violet laser and midwave UV.
Spodumene
Resplendor, Minas Gerais, Brazil
A thin and double terminated crystal. It is perfectly water-clear with a barely perceptible pinkish tint. Not really enough colored to call it Kunzite. Since the purple-pink color is caused by manganese, we can assume that this activator is also only marginally present here. This claim is supported by the correspondingly weak orange fluorescence of the manganese present.
Under midrange UV, about 312 nm, a slightly stronger blue fluorescence is visible. Probable activators here again: titanium, as [TiO6]8-, and defects in the oxygen bonds of the silicate group. You can easily apply the spectrum of the above kunzite to this specimen. Differences will be minimal.
Click on the photos to zoom in
Photos in resp. halogen, LW UV and midrange UV.
Photos in halogen light and 405 nm laser resp.
Spodumene
Svecofennian granitic pegmatites, Haapauluoma, Seinäjoki region Finland
A somewhat less aesthetic group of spodumene crystals with fine, elongated red tourmaline crystals. The fluorescence of this specimen is not very spectacular under the usual UV sources. Weak orange in long wave and weak blue in short and middle wave UV. However, under a 405 nm violet laser, the orange fluorescence is strong. This is caused by the presence of manganese, replacing aluminum. The photo in laser light was made with a Tokai Lutina filter to block the reflected laser light. The pegmatite fluoresces also weak white. As a result there was no need for additional illumination with white light.
Analcime
Afghanite
Agrellite with albite
Agrellite with thorite
Armstrongite
Benitoite
Beryl, var. goshenite
Beryl, var. emerald
Catapleiite
Dumortierite
Dumortierite
Hardystonite, willemite, esperite and calcite
Tremolite, var. hexagonite
Hiortdahlite
Laumontite on prehnite
Malayaite
Zircon
Willemite and calcite in tephroite
Stilbite
Esperite,Willemite and calcite
Chromian-tremolite (tremolite-Cr)
Natrolite
Spodumene, var. kunzite
Spodumene
Spodumene
Talc,pseudomorph after quartz
Talc after quartz, Johanneszeche, Göpfersgrün, Wunsiedel, Fichtelgebirge, Franken, Bavaria, Germany.
Resp. in white light and 365 nm LW UV (Convoy S2+)
Talc has completely replaced the mineral quartz here. However, the crystal form was perfectly preserved. It is very rare for a soft mineral like talc to replace a very hard mineral like quartz. This pseudomorphosis can be found in old collections dating back to the 19th century. For information about the fluorescence: Click on the prism.
Vesuvianite: Jeffrey Mine, Asbestos, Shipton Township, Richmond Co., Quebec, Canada.
Click the photos to zoom in.
A cluster of bright crystals of vesuvianite, some of which are slightly purple colored. This discoloration is caused by manganese ions that can replace both calcium and magnesium. The deeply purple colored crystals do not fluoresce due to concentration quenching, a phenomenon in which an excess of activator destroys fluorescence. The freestanding crystal is barely 2 mm high but is firmly playing along.
Click the prism to view the emission spectrum
Vesuvianite