Measured with: Ocean Optics Jaz spectrometer and a 312 nm LED light source.
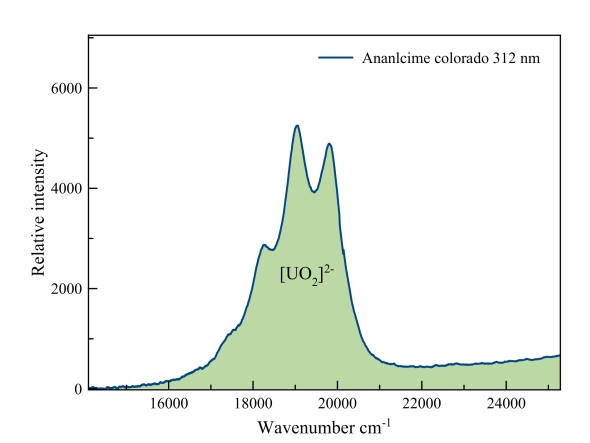
Analcime
Clearly the “fingerprint” of the uranyl fluorescence.
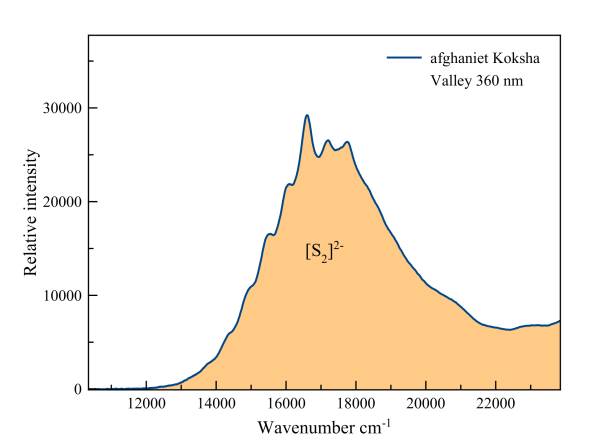
Afghanite
This fluorescence is "vibronic", the result of the vibration of the two sulfur ions around the middle of the covalent bond that unites them. With a constantly changing interatomic distance a series of possible transitions become real. Each of these transitions is expressed as a "hump" on the spectrum.
Agrellite + Albite
Move the mouse arrow over the image to see the spectrum of albite.
The fluorescence of aggrelliet is dominated by the emissions of trivalent cerium, samarium and dysprosium. Divalent manganese also makes a small contribution. Most likely, there are a number of other rare earths acting as activators but those emissions are drowned by the total fluorescence. The lion's share of the fluorescence takes place in the near UV. The deep red fluorescence of albite is mainly a result of trivalent iron which replaces aluminum. Possibly there’s also some divalent europium present.
Measured with: Ocean Optics Flame spectrometer, reflection/backscattering probe and a 360 nm LED light source.
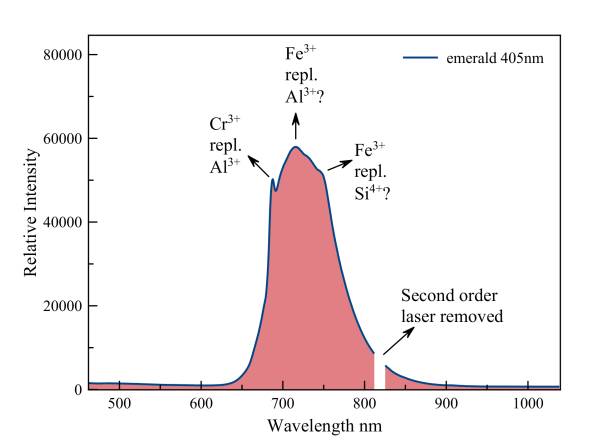
Beryl, var. Emerald
The sharp peak of fluorescent chromium, which replaces aluminum, leans against a much stronger emission of trivalent iron, which replaces both aluminum and Silicon. The tiny second order peak of the 405 nm laser was removed.
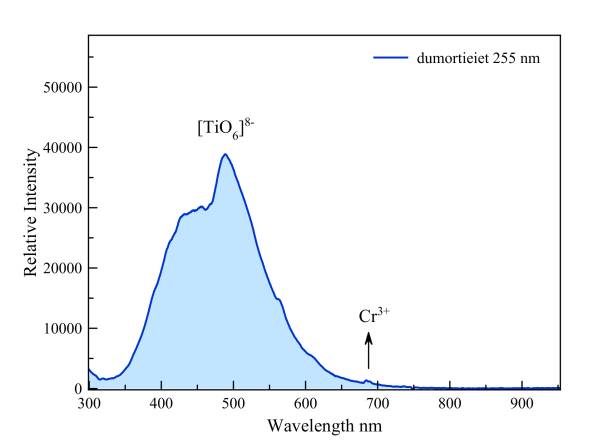
Dumortierite
According to Gorobets the blue fluorescence under short-wave UV is due to tetravalent titanium that, as [TiO6]8-, replaces aluminum (AlVI3+). There is also a possible emission in the red part of the spectrum by trivalent chrome, which also may replace Al3+. This emission is more strongly excited by laser light of 337 nm but it is still just visible at 681 nm and 688 nm under short-wave UV.
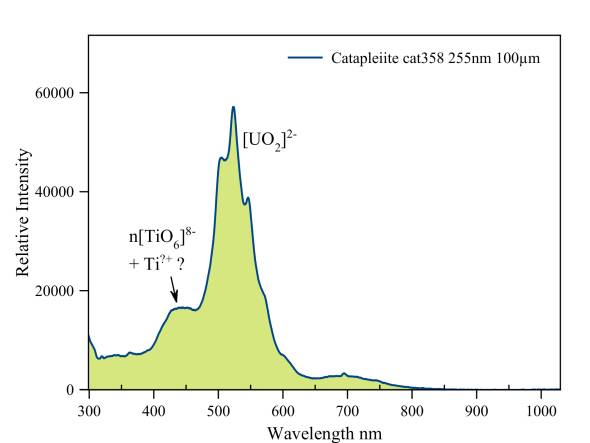
Catapleiite
This specimen has a "double" fluorescence. First of all, there is the blue fluorescence of trivalent and tetravalent titanium. Also the uranyl ion is present. This provides a green-yellow fluorescence. The predominant visual impression is a weak green fluorescence.
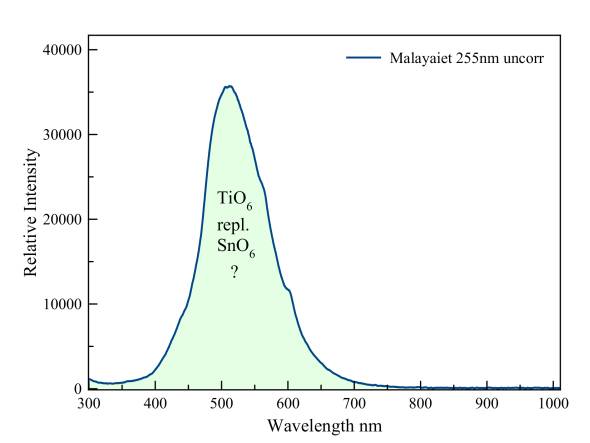
Malayaite is the tin-homologue of titanite. According to Gorobets the fluorescence is probably due to trivalent titanium, which replaces tin.
Measured with: Ocean Optics Flame spectrometer, reflection/backscattering probe and a 255 nm LED light source.
Measured with: Ocean Optics Jaz spectrometer, reflection/backscattering probe and a 405 nm laser diode.
Measured with: Ocean Optics Flame spectrometer, reflection/backscattering probe and a 255 nm LED light source.
Measured with: Ocean Optics Flame spectrometer, reflection/backscattering probe and a 255 nm LED light source.
Measured with: Ocean Optics Flame spectrometer, reflection/backscattering probe and a 255 nm LED light source.
Malayaite
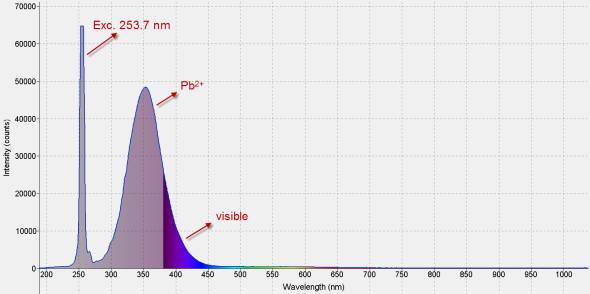
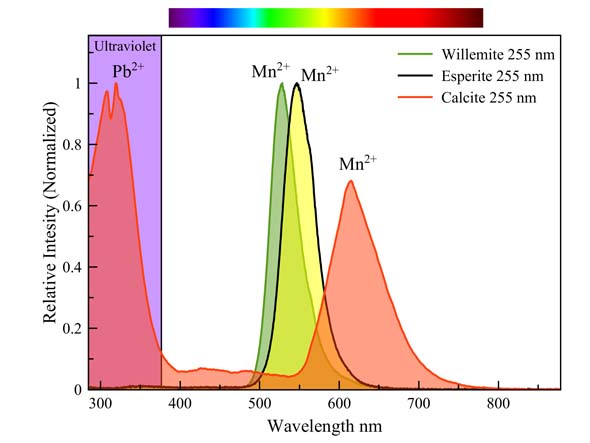
Less than 20 % of this fluorescence is visible to the unaided eye. The greater part of it happens in the mid-range ultraviolet, around 300 nm. Lead is the activator.
Measured with: Ocean Optics Jaz spectrometer and a 255 nm LED light source.
Hardystonite
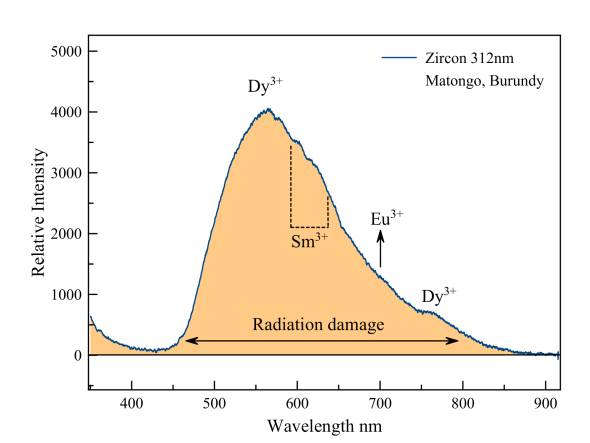
Zircon
The fluorescence of zircon is at its brigthest under midrange UV. This is caused by extensive damage to the crystal lattice by radioactive decay of isotopes of mainly uranium and thorium. Superimposed on the very broad emission peak of these crystal defects we see emissions of samarium and europium, dysprosium, all in trivalent state.
Ernst a. j. Burke wrote a very interesting article on zircon from Matongo in 1998. We publish it with pride and with many thanks to the author, who in the meantime became a member of our society. You may download the text in PDF format by clicking on the icon to the right.
Measured with: Ocean Optics USB 2000 spectrometer and a 312 nm LED light source.
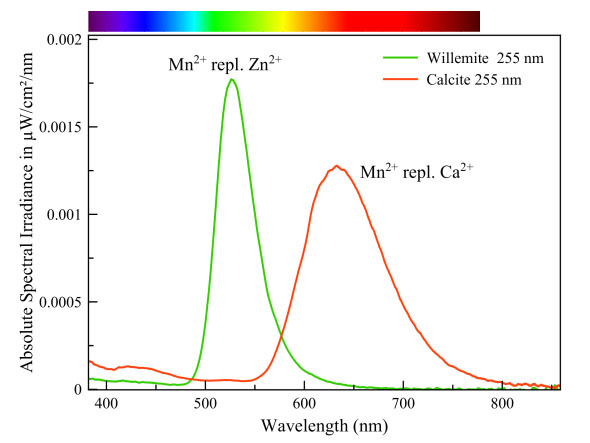
Manganese can cause a whole range of fluorescent colors. The color depends on the way the manganese ion is embedded in the crystal lattice. More specific: the coordination with the surrounding oxygen atoms. Those oxygen atoms exercise, due to their negative charge, an electrostatic pressure on the outer orbitals of the manganese ion. That pressure affects the amount of energy involved in the transitions in the manganese ion. Manganese in calcite is surrounded by 6 oxygen atoms and its transition energy is therefore smaller than in willemite in which manganese is surrounded by only 4 oxygen atoms. This is why manganese fluoresces red in calcite and green in willemite.
Willemite & calcite
Measured with: Ocean Optics Flame spectrometer, reflection/backscattering probe and a 255 nm LED light source.
Calibrated with Ocean Optics HL-2000-CAL
Calcite
Willemite
Esperite, Willemite & calcite
Manganese causes a different fluorescence color in three minerals on the same specimen (see text of spectrum above). It is striking that the greenish-yellow esperite differs only very slightly in color from the green willemite. Both spectra overlap for the most part. Many people have trouble distinguishing between these colors. Manganese needs no co-activator in willemite and esperite, but it does require one in calcite. This calcite contains some ppm lead, which fluoresces strongly in the UV between 280 nm and 350 nm. This wavelength range is extremely suitable for the excitation of manganese in calcite.
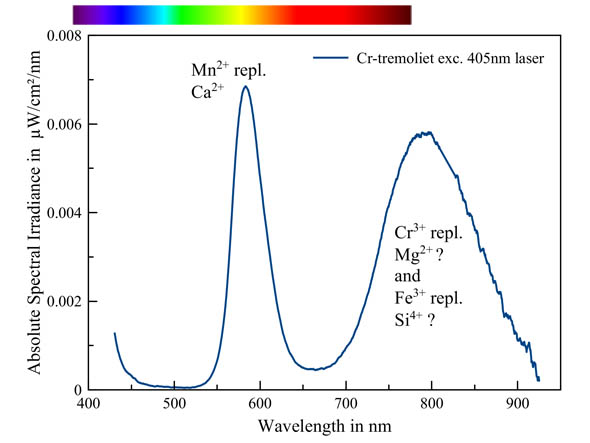
Chromium is a well-known activator that produces a red fluorescence in many minerals. In this tremolite-(Cr), the orange fluorescence is caused by manganese, the most common activator in tremolite. However, there is still a strong second band present in the Red and infrared part of the spectrum. This could be caused by chromium replacing magnesium and/or iron replacing silicon. However, literature remains vague, except for the involvement of manganese.
Chromium-tremolite - tremolite-(Cr)
Spodumene, var. Kunzite
Spodumene is a lithium aluminum silicate. Lithium is a small ion, so there are not many elements with the appropriate dimensions and load to replace it. However, silicon and silicate groups are easily replaced by other elements (and anions) as well as aluminum.
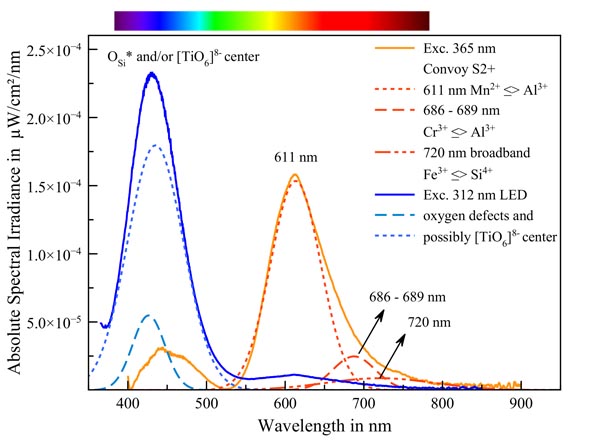
Spodumene fluoresces in two colors depending on the excitation energy. Under the powerful 365 nm UV bundle of the Convoy S2+ lamp, we see a strong orange emission. Mathematical decomposition of the spectrum in its Gaussian components yields three distinct peaks. These peaks lie at exactly the values that Gorobets and Rogojine published. (Luminescent Spectra of Minerals: Reference Book, Boris S. Gorobets & Alexandre A. Rogojine).
| Peak wavelength |
replaced element |
replacing element |
| 611 nm |
Al3+ |
Mn2+ ** |
| 686 nm - 689 nm |
Al3+ |
Cr3+ |
| 720 nm (broadband) |
Si4+ |
Fe3+ |
Since the deconvolution of the spectrum in its Gaussians is compliant with the measured curve with acceptable accuracy (adjusted R ^ 2:0.9955) we can establish these activators with confidence.
* * Gorobets mentions Mn2+. Other sources are talking about Mn3+, which is more accurate in terms of charge.
The blue fluorescence in 312 nm UV (the middle section of the UV range) is not yet explained with certainty. Gorobets speaks about oxygen defects, specifically: non-interconnected oxygen in the silicate group. These defects cause a blue fluorescence in many silicates but the location of the peaks in the spectrum is not quite right. The blue fluorescence is also somewhat too intense to attribute it only to oxygen defects. A large part of it may be caused by the [TiO6]8- group which, as an anion, can replace silicate groups. This [TiO6]8- group is responsible for the blue fluorescence of, for example, benitoite and dumortierite. Gaft & al. also point in that direction but it has not been thoroughly investigated.
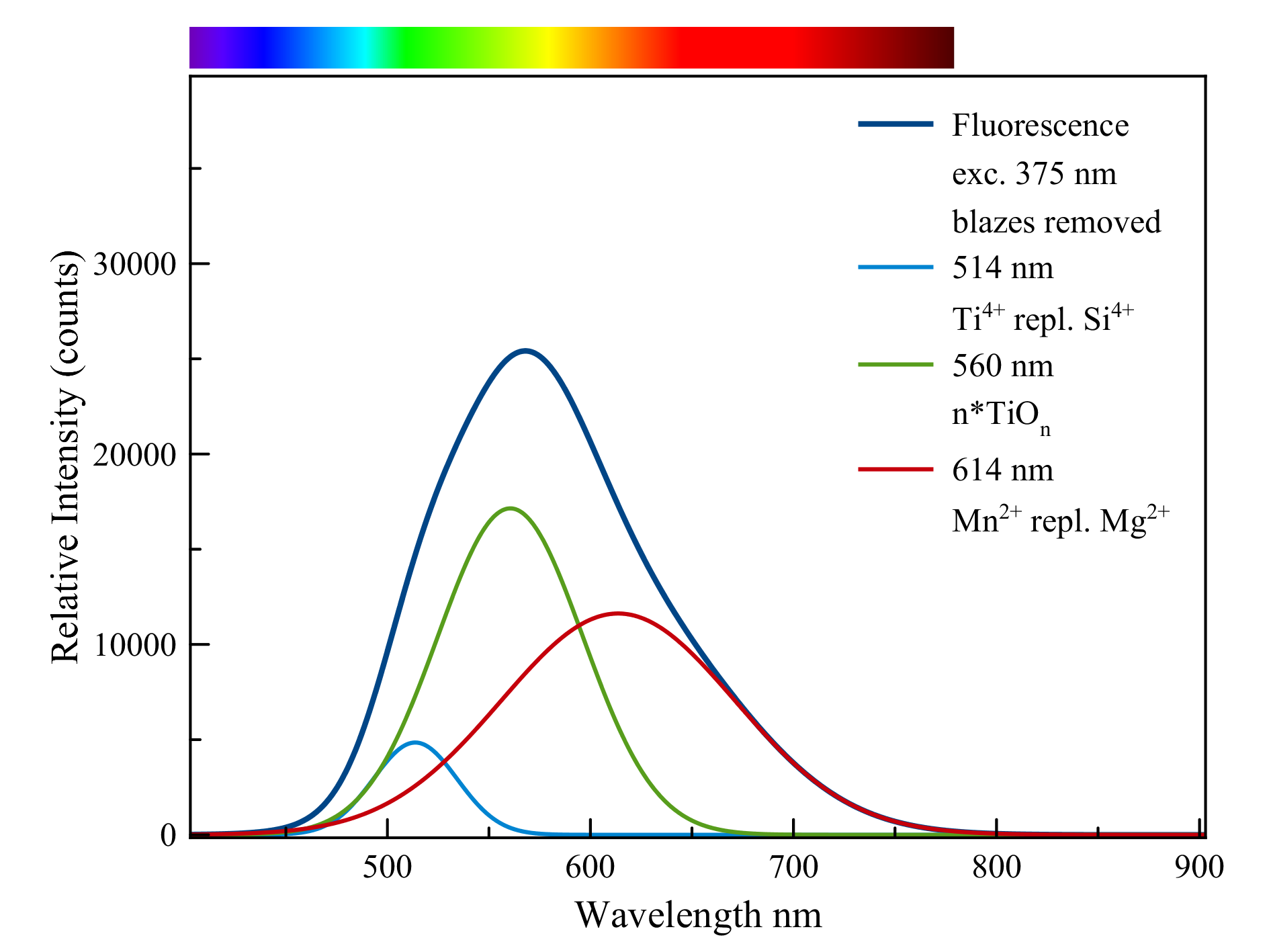
Talc,pseudomorph after quartz
The fluorescence can be divided into three main contributions:
1) titanium, Ti4+,as a replacement for silicon, Si4+,a green emission.
2) clusters of the titanate anion, n*(TiOn))n- which light up in the green and yellow
area.
3) Manganese, Mn2+, which replaces magnesium in talc with an orange to red
fluorescence as a result.
The three colors together form the dirty white color that is visible on the crystal ribs.
The crystal surfaces themselves appear to fluoresce a weak brown. However, this is
due to the dirty white light that is filtered through the rust-brown surface layer. The
fluorescence is weak to very weak under normal UV lamps but much stronger under
the light of the Convoy S2+ LED lamp
Talc after quartz, Johanneszeche, Göpfersgrün, Wunsiedel, Fichtelgebirge, Franken, Bavaria, Germany.
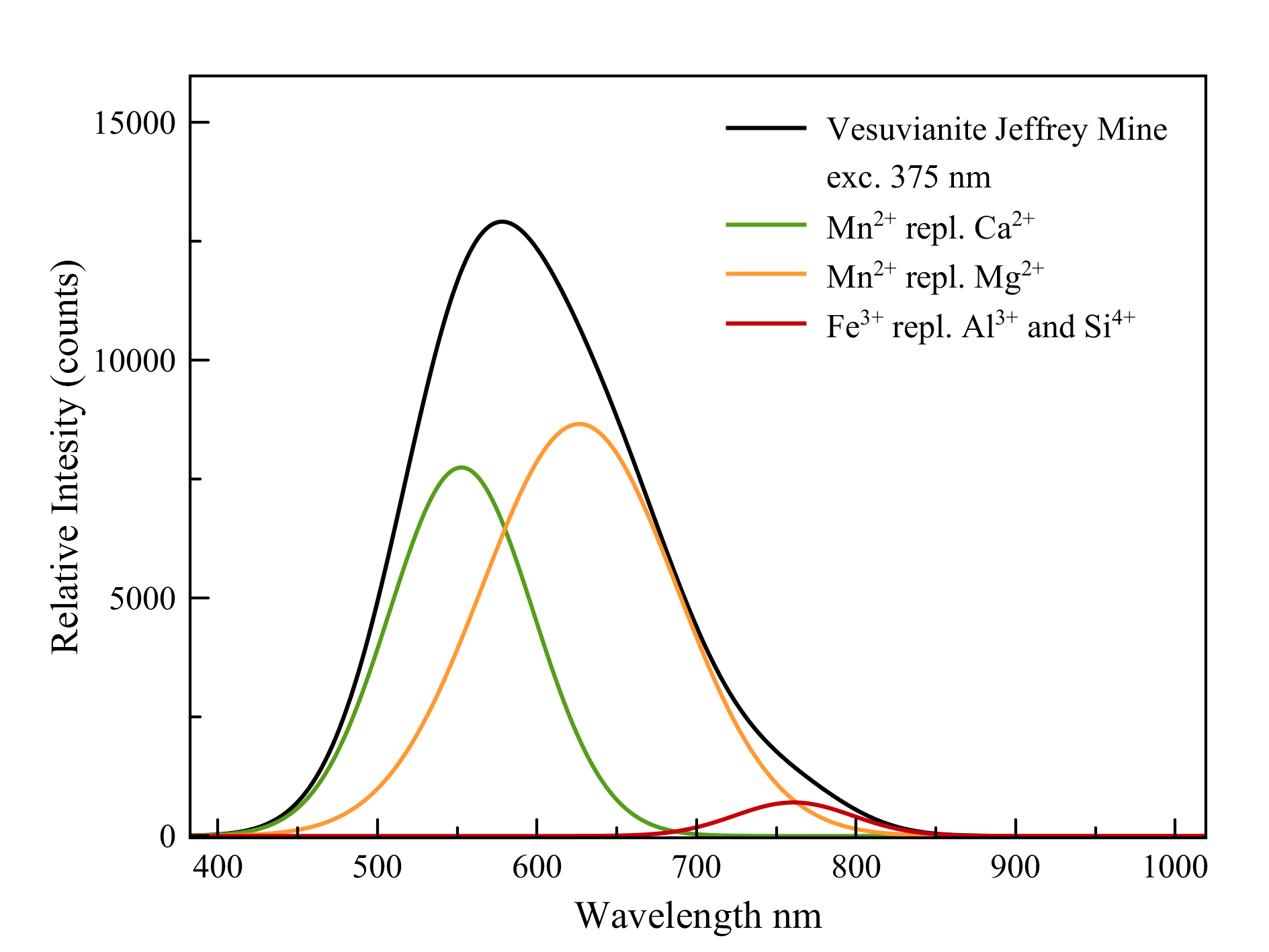
Vesuvianite
Ca10Mg2Al4(SiO4)5(Si2O7)2(OH)4
As the (simplified) formula shows, vesuvianite contains two ions that can be replaced by manganese: calcium and magnesium. The sites in which these ions are located are slightly different in size and environment. As a result, manganese in these sites will also fluoresce slightly differently. Both emissions do overlap and cause a wide peak in doing so. There is also some trivalent iron that loves to replace aluminum and even, to a lesser extent, silicon. This causes a deep red fluorescence.
Measured with: Ocean Optics Flame spectrometer, reflection/backscattering probe and a 375 nm LED light source.
Calibrated with Ocean Optics HL-2000-CAL