Fluorescence is a form of luminescence in which a substance is irradiated with light of a certain wavelength, upon which the substance itself will emit light of a (usually) longer wavelength.
Absorption of photons with a certain wavelength in such case leads to the almost immediate emittance of photons with a usually longer wavelength.
The emittance of light by the irradiated substance ends almost instantaneously after the radiation source is turned off.
This distinguishes fluorescence from phosphorescence, in which the emittance of light continues for some time after the source of the exciting radiation has been turned off.
In the traditional sense, fluorescence can only occur when a few conditions are met: firstly, the exciting radiation must be of electromagnetic origin, i.e. photons.
Certain substances fluoresce when they are irradiated with electrons.
However, this is not true fluorescence but luminescence, more specifically cathodoluminescence.
Secondly, the emitted radiation must be in the visible range of the spectrum.
However, to make things easier, we also speak of fluorescence when the emitted light is in the ultraviolet or infrared range of the spectrum.
Very few minerals are known that are the same colour in both daylight and under ultraviolet light, e.g. ruby.
In most minerals however, the colour in daylight differs considerably from that under ultraviolet light.

Corundum, Prîlep, FYROM Macedonia. Photo in LW UV. Photo & collection: A. Emmermann
Other forms of luminescence are:
Thermoluminescence: when a substance becomes luminescent when it is heated;
Cryoluminescence: when a substance becomes fluorescent only at very low temperatures;
Bioluminescence: light that is emitted by bacteria and/or fungi (rot) or slow oxidation in life forms (e.g. fireflies);
Chemoluminescence: light that is caused by chemical reactions, usually slow oxidation;
Triboluminescence: light that is caused by the scratching of a mineral substance;
Radioluminescence: light that is caused by submitting a substance to radioactive radiation;
Sonoluminescence: light that is caused by collapsing vacuoles as a result of ultrasound in a liquid under pressure;
Cathodoluminescence: light that is caused by radiation of a substance with an electron beam;
Fractoluminescence: light that is caused by the crushing of quartz crystals;
Phosphorescence: luminescence that persists after the source of the exciting radiation has been turned off.
What is light?
Light is that part of the electromagnetic spectrum that we are able to perceive with our eyes, in other words, the part we can see.
This visible part of the spectrum ranges from around 380nm to around 780nm.
Particles of light are called photons.
These are emitted when a charged particle, an electron or a proton, loses energy.
On the other hand, when a charged particle is struck by a photon, this particle will gain energy.
The energy content of a photon is directly proportional to the amount of energy the charged particle which emitted the photon has lost.
The more energy a photon has, the shorter its wavelength is.
Fluorescence and minerals
Some minerals will emit light when they are placed under an ultraviolet light that is switched on. The emitted light usually completely differs in colour from that of the mineral in daylight.
Some minerals even fluoresce when they are radiated with (visible) bluish green light.
Ruby and manganocalcite are well-known examples of this phenomenon.
A number of wavelength ranges that can be used as exciting radiation can be distinguished:
- visible light, usually blue or green;
- long wave ultraviolet: 320nm to 420nm.
This is obviously somewhat arbitrary.
All wavelengths of light above 380nm can be regarded as violet or even blue light;
- Mid wave ultraviolet: 280nm to 320nm (also called UV-B);
- Short wave ultraviolet: usually the 253.7nm spectral line of mercury is used.
In principle, short wave ranges from 100nm to 280nm.
Some minerals react to all light sources in the same way, with a difference in the intensity of the fluorescence at best.
Other minerals react differently to different wavelengths of ultraviolet light, or only fluoresce under a certain wavelength.
Atoms and light.
Atoms roughly consist of two parts: the nucleus and the electrons.
The nucleus consists of protons and neutrons.
The protons are positively charged particles.
For each proton in the nucleus, the atom has a negatively charged electron in its mantle.
The negatively charged electrons are electrostatically attracted to the positive charge of the atom’s nucleus.
Consequently, the electrons prefer to stay as close to the nucleus as possible.
This is called the “ground state” of the atom in question.
Electrons cannot move around freely within the electron mantle.
They are bound to orbits (orbitals) of which the energy content (the distance to the nucleus and the speed of the electron: the orbit must comprise a round number of wavelengths of the electron) is very precisely fixed for every electron of every element.
When an electron is struck by a photon of which the energy is exactly equal to the difference in energy of the electron’s current orbit and a higher allowed orbit, the electron will jump to that higher orbit.
This jump happens instantaneously and the electron will not take up any intermittent position in between both orbits.
The photon that causes this jump is called the exciting radiation.
The atom is now no longer in its ground state, but has now been “excited”.
An atom cannot stay in an excited state for long.
Within a very short time, usually within a few millionths of a second, the electron returns to its original orbit, as close to the atom’s nucleus as possible.
The atom thus returns to its ground state.
On its way down to its original orbit, the electron can (but doesn’t have to) make a short “pit stop” at every allowed orbit.
The return to the original orbit can thus be divided into a few shorter jumps.
During every jump, the electron emits a photon of which the energy content equals the difference in energy of the orbit it has reached and the previous orbit.
One or more of these jumps can result in a photon within the visible spectrum.
The atom then fluoresces.
Causes of fluorescence in minerals.
A number of different causes of fluorescence are known:
• Inclusions of fluorescent substances: sometimes substances that are intrinsically fluorescent are included in minerals during their crystallisation.
This causes the minerals themselves to become fluorescent as well.
The included substances can e.g. be clay particles or other fluorescent minerals, such as finely distributed scheelite.
Certain organic acids can also be included in the mineral, such as humic acids and other waste products of organic origin.
Very finely divided scheelite dust is the cause of the blue fluorescence of this colorless beryl, Pingwu mine, Hunan, China. Collection and photo: A. Emmermann, photo under SW UV.
Calcite, Mont-sur-Marchienne, Hainaut, Belgium. Organic compounds such as humic acids can cause fluorescence. Collection & photo: A. Emmermann, photo under SW UV
• Intrinsic fluorescence: some minerals are intrinsically fluorescent.
The fluorescence is not caused by contamination, but an essential consequence of their chemical composition.
Scheelite and most of the fluorescent uranium minerals fall into this category.
Most borates and a number of lead minerals are also considered to be intrinsically fluorescent.
Autunite, Autun, France. Photo & collection: A.Emmermann, Photo in Long Wave UV.
Cerussite, Mibladen, Midelt, Morocco.
Under LW-UV
However, the fluorescence mechanism of cerussite, a lead mineral, is still not clear.
It shows many similarities with that of certain baryte specimens, in which monovalent silver turns out to activate the fluorescence.
Cerussite’s yellow fluorescence used to be regarded as an example of intrinsic fluorescence of a lead mineral. Today however, this assumption is questioned.
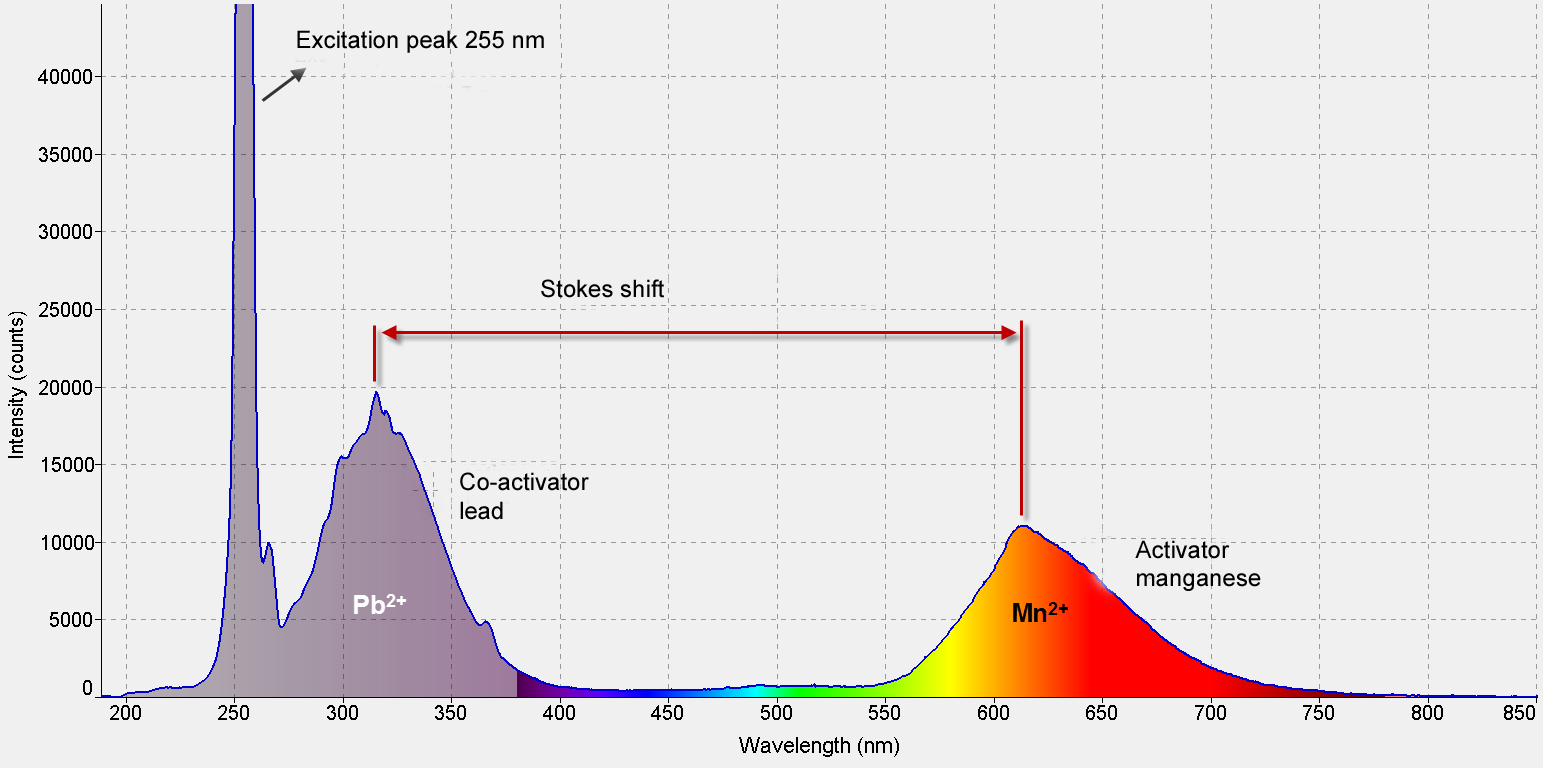
• Activators and co-activators: in the crystal lattice of a mineral, a number of atoms of the mineral can be replaced by other ions.For example, manganese can easily replace calcium in calcite.
The replacing ions can then, when they possess incompletely occupied electron shells below the valence shell, cause fluorescence. These ions are called "activators". Manganese is capable of autonomously generating fluorescence in e.g. willemite.
In this case, manganese replaces zinc atoms and as a silicate, the manganese is able to absorb a fairly wide range of ultraviolet radiation. However, as a carbonate, in calcite, manganese is not capable of absorbing short wave ultraviolet light directly. Calcite which is only contaminated by manganese will therefore not fluoresce.
However, when a little bit of lead or trivalent cerium is also present in the calcite, these ions will absorb the ultraviolet light and convert it into energy which can be absorbed by manganese. In other words, these ions fluoresce and emit mid-range UV: around 310nm.
At these wavelengths, the manganese (as a carbonate) can fluoresce. These ions, which serve as “pre-ignition” of the fluorescence, are called co-activators or primers.
In fact, this is a kind of "multistage" fluorescence.
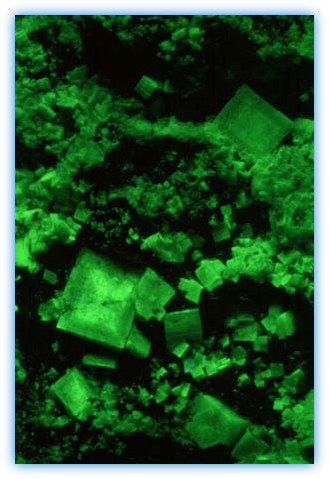
Short wave UV causes fluorescence of lead in calcite from Franklin, New Jersey, USA. This fluorescence in turn causes the manganese ions to fluoresce.
Calcite, Rio Grande do Sul, Brazil.Activator: manganese and lead. Photo under MW UV
• Errors in the crystal lattice: missing or incorrectly placed atoms can lead to errors in a crystal lattice.
These errors disturb the electrostatic balance at their locations.
This in turn can lead to fluorescence centers like F-centers and M-centers.
Fluorite, Seilles, Namur, Belgium. Fluorescence is caused by crystal defects Photo: A. Emmermann, collection: Richard Loyens, photo in LW UV
Ions can cause errors in a crystal lattice because their sizes differ slightly from the ions they replace. For example, strontium can replace some calcium in gypsum or baryte, causing disruptions in the regularity of the crystal lattice.
These ions are called “promoter ions”. They do not fluoresce themselves, but they “promote” fluorescence centres within the crystal.

About "quenching" and “fluorescence killers”
The specimen pictured has no intrinsic value whatsoever.
The sole purpose of this image is to show how the presence of iron oxides can “spoil” even the strongest fluorescence.
The specimen originates from the quarry of Mont-sur-Marchienne, Hainaut Province, Belgium.
It is a geode in limestone with countless small calcite rhombohedra which fluoresce bright white.
Pyrite is also abundant in the quarry.
The rust spots on the specimen are caused by oxidation of this pyrite.
By moving your mouse over the image, the same specimen is shown under ultraviolet light.
The fluorescence of the calcite can be seen clearly, except in the places where the rust spots are.
This phenomenon can be fairly easily explained: plant remains in the soil above the limestone subsoil rot, generating complex humic acids.
These acids, together with the carbon dioxide in the atmosphere, slowly dissolve the limestone and “percolate” deep into the subsoil.
Small cracks and fissures are widened, sometimes developing into vast cave systems.
On its way down, the acidic water becomes more and more saturated with the calcium carbonate it dissolves from the limestone.
When saturation is complete, calcium carbonate will again crystallise from the solution as calcite.
The humic acids and possibly other organic substances that were encased in the limestone will be included in the calcite crystals, which causes them to strongly fluoresce.
Iron oxide is fairly mobile as well, as a gel which originates from the oxidation of pyrite.
It is also included in the calcite crystals.
This has two consequences: firstly, iron oxide readily absorbs UV radiation and blue light.
This causes very little light of the right wavelengths to cause calcite to fluoresce to penetrate the crystals.
This weakens the fluorescence considerably.
Secondly, the light of the already weakened fluorescence needs to travel back up through the dark brown crystal to exit.
Therefore, it is understandable that iron is known as a fluorescence “spoiler” or even “killer”.
Moreover, you can clearly see that the fluorescence on the edges of the rust spots shows both a colour and brightness gradient.
This is simply the white fluorescence which is filtered by the brown crystals.
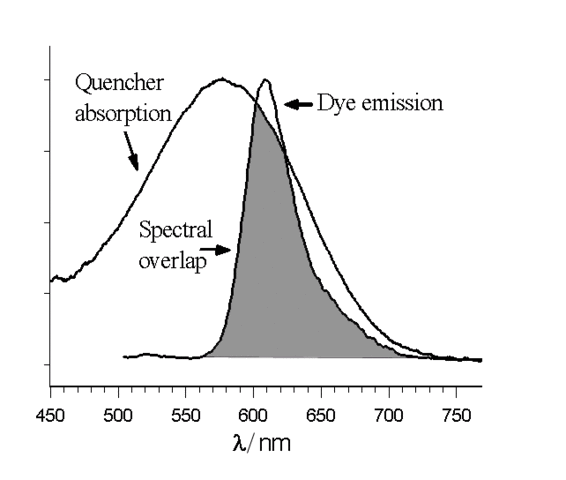
A similar phenomenon can be observed in e.g. cupro-powellite.
This variety of powellite is coloured somewhat green by copper which partially replaces calcium. Powellite strongly fluoresces yellow.
However, when we take a closer look at the fluorescence spectrum, we can see that it contains a fairly large amount of green and red light.
The green copper ions filter out the red light and what remains is a green fluorescence.




Move mouse arrow over photo to see fluorescence. Calcite geode, Mont-sur-Marchienne, Hainaut, Belgium
"Regular" powellite: yellow under SW-UV. Largest Crystal = 4 cm high. Mahodri near Nasik, Maharashtra State, India
Cupro-powellite under SW-UV
Jardinera #1 Mine, Inca de Ore, Atacama, Chili
The diagram shows how an emission peak and absorption peak may overlap. Virtually all fluorescence is immediately absorbed here.
Fig. by Jan Luca & Magnus Manske
Of course, a simple physical shielding of the UV light can prevent fluorescence.
There are abundant examples of hematite dust in calcite or galena dust in cerussite.
Atoms and luminescence
The return of excited atoms to their ground state in jumps explains Stokes’ law, which states that in fluorescence the wavelength of the emitted light is always longer than that of the exciting radiation, with a few exceptions (anti-Stokes law).
Sir George G. Stokes is generally recognised as the discoverer (1852) of fluorescence caused by ultraviolet light.
He called the phenomenon fluorescence because he first observed it in fluorite (the famous fluorite of Weardale, England).
Therefore, fluorescence has nothing to do with the element fluoride, which many people seem to think.
Sometimes electrons are treated a bit too roughly by the exciting radiation, and they are completely “detached” from their respective atom.
These isolated electrons are often captured in vacancies within a crystal.
Then they can no longer return to their ground state on their own and have to wait until they get a little nudge.
They can get this nudge from other photons and/or lattice vibrations (phosphorescence), radioactive radiation (radioluminescence) or strongly increasing lattice vibrations due to a raise in temperature (thermoluminescence).
Isolated atoms, i.e. atoms which are not bound, will rarely show fluorescence (usually rarefied and already partially ionised gases).
However, the complexity of the electron structure increases when atoms are chemically bound to each other.
The number of possible jumps of the electrons BELOW the valence electrons proportionally increases as well.
That is why we usually find fluorescent substances in the realm of chemical compounds, both organic and inorganic, and rarely (if ever) in the elements.