Spectra Sulfates - Molybdates - Tungstates

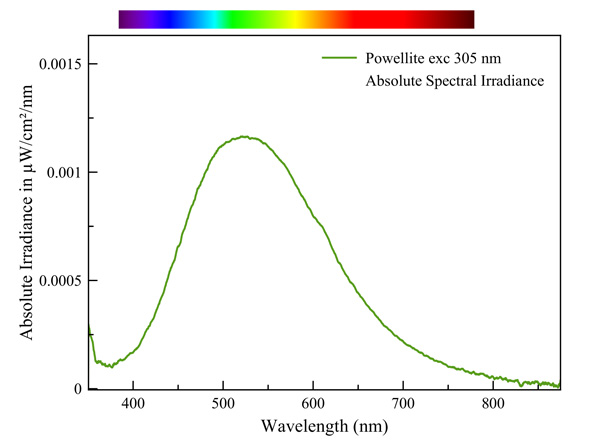
Powelliet
This is an intrinsic fluorescence, analog to that of scheelite. It is typical of complex anions with a central metal ion that is surrounded by oxygen ions. The general term for this mechanism is 'charge transfer', in which electron transitions happen between oxygen and the central metal ion. Examples of such anions are: WO42-, MoO42-, VO43-, TiO68-, UO22+, etc... This mechanism invariably involves extremely broad emission bands.
Measured with: Ocean Optics Flame spectrometer, reflection/backscattering probe and a 255 nm LED light source.
Calibrated with Ocean Optics HL-2000-CAL
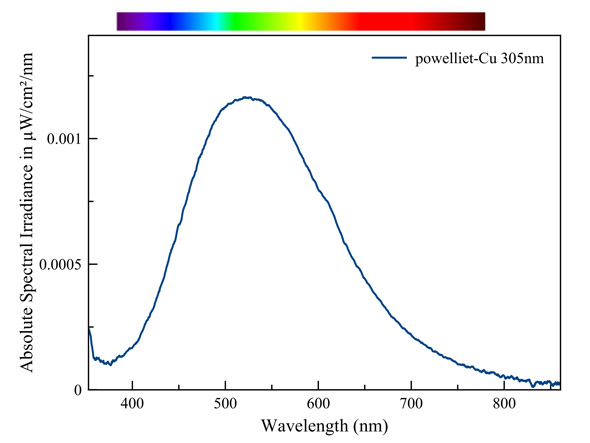
Powelliet - Cu (Cupropowelliet)
The fluorescent color appears, just like the color under ordinary light, somewhat greener than that of pure powellite. The difference is however very small when we compare the spectra. It's a similar effect as when sunlight shines through a green colored glass. A small amount of the red light in the powellite fluorescence is absorbed by the copper. As a result we perceive this fluorescence as a tad greener. However, the fluorescence mostly happens at the surface of the crystals. This renders the path length of light emission through the colored crystals and, as a result the absorption of red light, very small.
Measured with: Ocean Optics Flame spectrometer, reflection/backscattering probe and a 255 nm LED light source.
Calibrated with Ocean Optics HL-2000-CAL
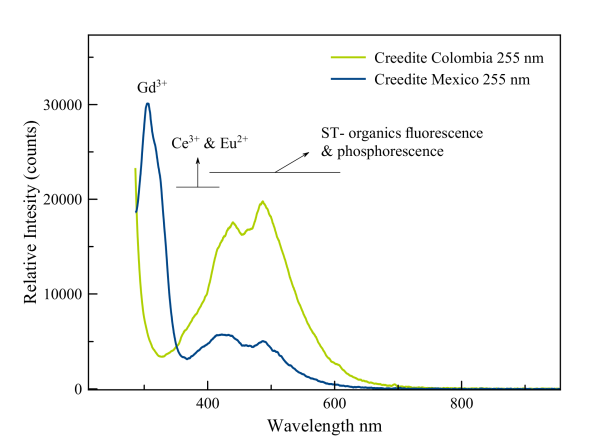
Creedite
A strong peak is seen in the region just above 300 nm. Gorbets and Gaft both assign that to trivalent gadolinium. This massive fluorescence in the midrange UV triggers two other activators that appear to be present: Ce3+ and Eu2+. The fluorescence of cerium is seen in the violet and probably in the tailing of the gadolinium peak. Divalent europium causes a broad peak that runs from 380 nm to 450 nm. It thus overlaps with the emission of the humic acids. The comparision of the spectrum with that of a Bolivian specimen demonstrates the importance of the rare earth elements in this blue fluorescence.
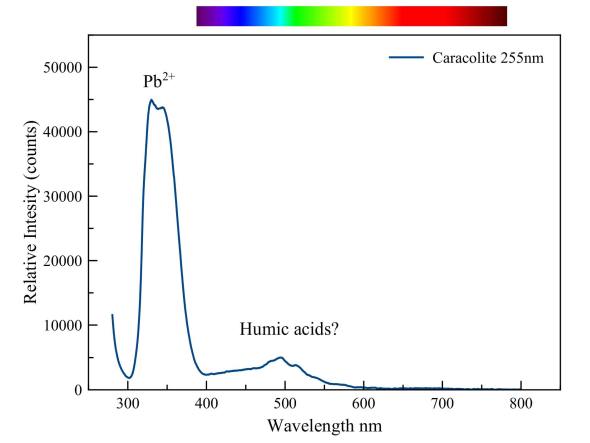
Caracolite
The very strong emission peak of lead dominates this spectrum of caracolite under short-wave UV. The violet blue fluorescence that we see with the naked eye is but a few percent of the very strong fluorescence which manifests itself in the UV. A weaker emission is visible in the blue-green part of the spectrum. Presumably, it is due to some organic material. That lead can cause a strong fluorescence even in glass is demonstrated in the picture below.
Bohemian crystal "bonbonnière" under halogen and SW-UV light.
Baryte on calcite matrix, Devon
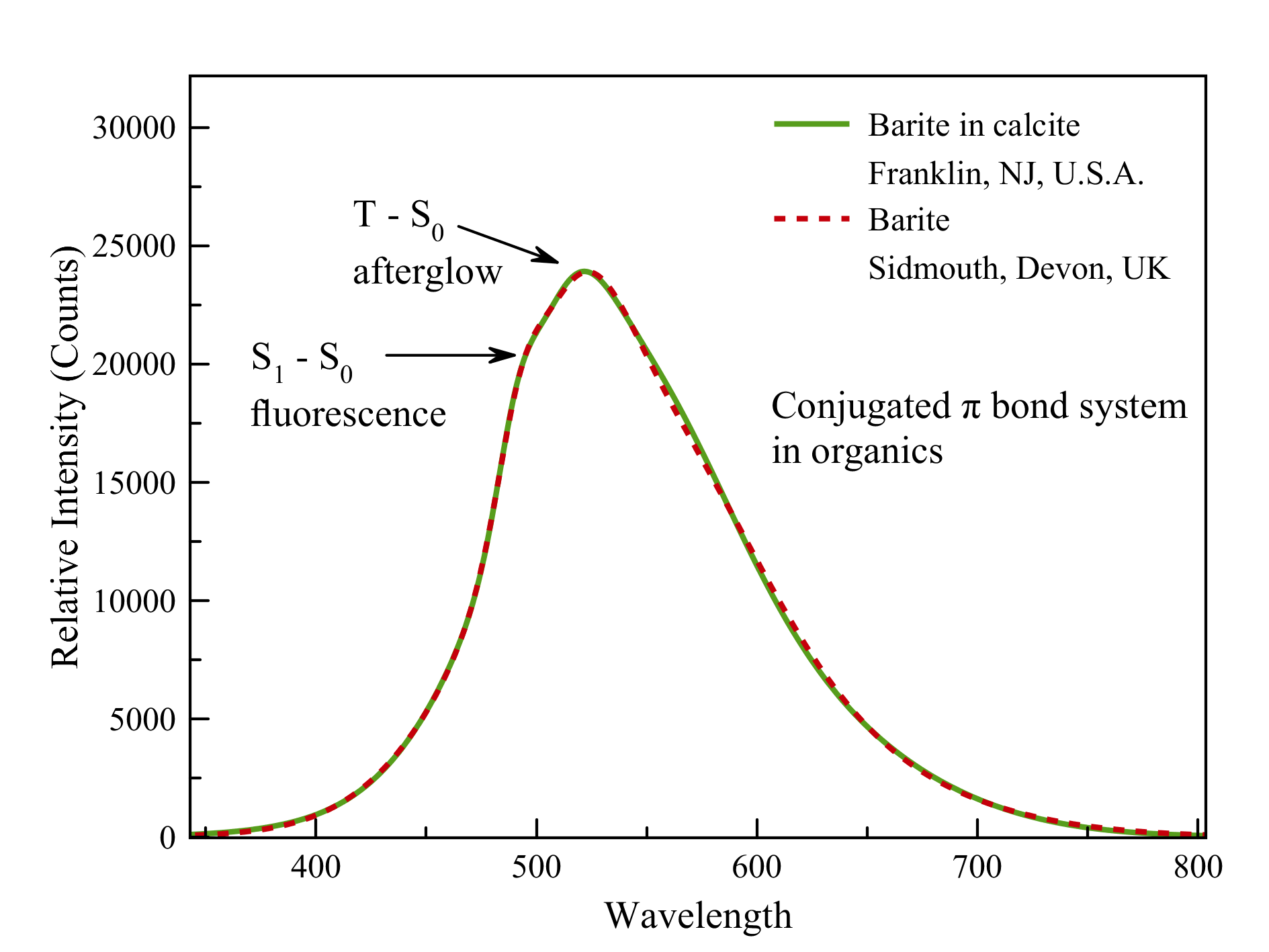
Much to my surprise, the spectrum of this Baryte from Devon exactly matches that of the famous baryte found in red fluorescent calcite from the Sterling Hill mine, Ogdenburg, New Jersey (see below).
.
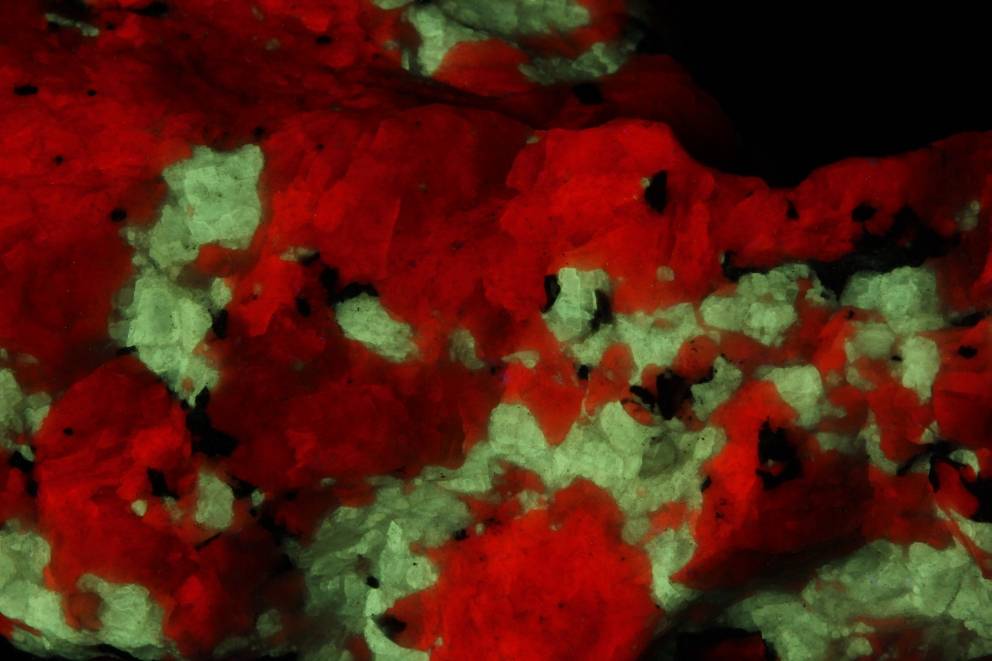
At first glance, this baryte fluoresces a bit yellowish, unlike the baryte from Devon, which fluoresces a whitish green. The explanation for this may have to be sought in the bright red ambient fluorescence in which the Sterling Hill baryte is set, which is misleading to our eyes. The spectrometer shows that both emissions are completely identical! The cause lies in the presence of organic matter containing conjugated π-bonds.
Baryte in calcite in SW-UV
Sterling Hill Mine, Ogdenburg, NJ, USA
These bonds are excited by UV and exhibit both fluorescence (S1-S0 transitions or singlet-singlet transitions) and afterglow with a slightly larger wavelength (T-S0 transitions, triplet-singlet transitions). This phenomenon originates in a quantum mechanical phenomenon called 'spin-orbital coupling' and is, in its turn, controlled by Werner Heisenberg's Uncertainty Principle.
Scheelite Yaogangxian mine
Click on spectra to enlarge
The Yaogangxian mine in the Yaogangxian W-Sn ore field, Yizhang Co., Chenzhou, Hunan, China, lies in a contact halo of a granite intrusion into alternating layers of sedimentary sandstone (cambrian-devon) and limestone (jurassic).
The genesis and geological setting of a mineral as a chemical determines the inventory of rare earths that can replace calcium in it. Scheelite can arise in a number of ways: contact metamorphosis and skarns, high temperature hydrothermal veins and greisen, granitic pegmatites, and medium temperature hydrothermal veins. It is also found in alluvial deposits but of course it did not originate there.
Large intrusions (batholites) give rise to hydrothermal formation of minerals. The type of magma that makes up this intrusion is important. Intrusions of magma derived from crustal volcanism contain little rare earths. If they are present, they often come from the surrounding rock in the contact halo. Intrusions of mantle magma do contain higher concentrations of rare earths.
The spectral analysis of scheeliet can say a lot about the way it is created. Usually we see, under short wave UV, a typical blue fluorescence. The top of that blue emission band (at 450 nm - 500 nm) is just the right wavelengths to excite the rare earths praseodymium and samarium. So they start to fluoresce by stealing energy from the blue fluorescence of the complex ions [WO4]2- . This 'stolen' energy becomes visible as a ‘dent’ in the blue emission peak of the scheelite under short wave.
Under mid-range and long wave UV, this blue peak is absent and we only see the important emissions of a whole range of rare earths.