Spectra oxides - hydroxides

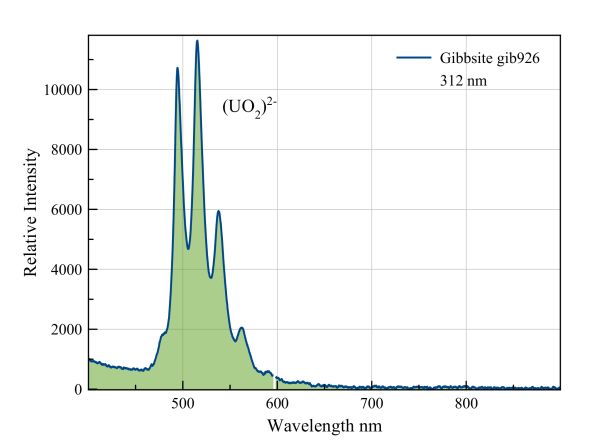
This is clearly a "fingerprint" of the uranyl ion. The green fluorescence is typical of minerals that are contaminated with this ion.
This is a "mouseover" spectrum. Move the mouse arrow on this spectrum to switch between the red and orange fluorescent emission of this peculiar corundum. The orange fluorescence corresponds to what Gorobets proposes for diaspore in which divalent manganese is included. There should still be a compensation found for the charge difference between Mn2 + and Al3 +. From different sources state that the corundum from Prileb contains a lot of diaspore. Sufficiently so, to cause a special light reflection the that has been named "diasporescence". The Orange fluorescence would thus not originate in the corundum, but in the embedded diaspore. The links below contain more information.
Document 1
Document 2 (will be downloaded as PDF)
Measured with: Ocean Optics JAZ spectrometer with backscattering/reflection probe and a LED light source from 312 nm.
Measured with: Ocean Optics Flame spectrometer with backscattering/reflection probe and a LED light source from 312 nm for the orange fluorescence and a 360 nm LED source for the red fluorescence.
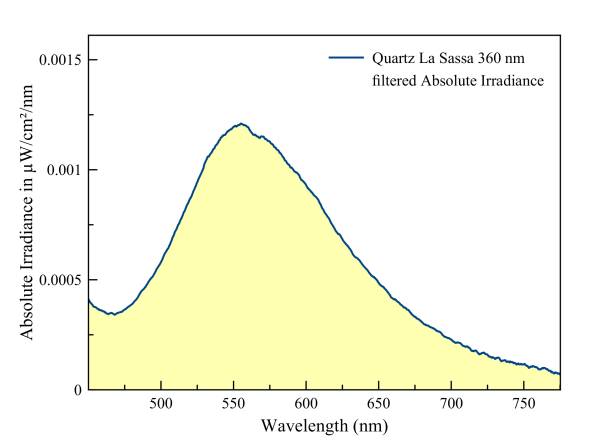
Quartz, yellow fluorescence
A broadband spectrum with very few details. There are some very small peaks visible, just hardly above the noise level. Some rare earth emissions and possibly some uranyl? However, the cause of the strong broadband emission still remains unexplained.
An extensive article of this locality and fluorescence was published in 'Journal Of The Fluorescent Mineral Society 2012', Volume 32: 'A preliminary report: The glowing quartz from La Sassa, Tuscany, Italy, door Antonello Dallegno and Guido Mazzoleni.
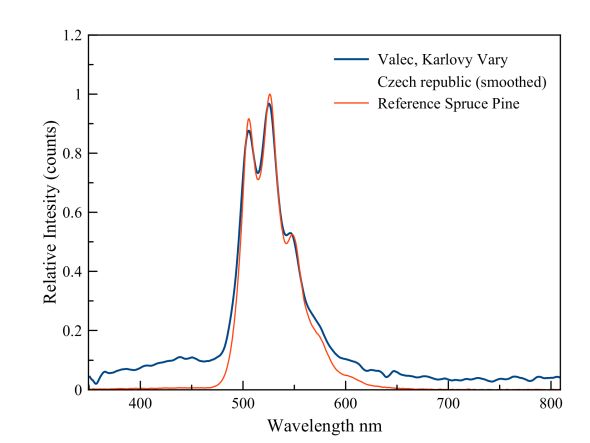
Hyalite or glass opal, Valec
This hyalite specimen is clearly contaminated with the uranyl ion. Hyalite is Opal-AN (amorphous Network). It does not have the same buildup of micro-spheres of cristobalite or tridymite, such as noble opal species do. It’s the size of these micro-spheres that determine the color of the "opalescence". Hyalite is just a silica gel that has formed a silica network while losing water. This is why hyalite is colorless and transparent when pure. An extensive article about Valec's Opal has recently appeared in Lapis. See Lapis 1/2018 P 10-21. Lapis too mentions UO22+ as a cause of the green fluorescence. Because the uranyl ion can show quite a few different spectra, depending on the medium in which it is located, I have measured a reference spectrum of a hyalite specimen from Spruce Pine. It has been established that it indeed contains uranyl. The similarity with the Valec specimen is obvious.