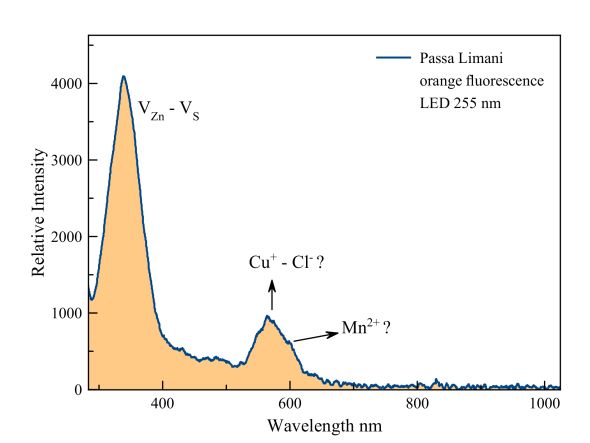
This specimen is just 1 cm in size. The emission of the minute orange dots is correspondingly weak but still measurable. The orange fluorescence is most likely caused by manganese replacing zinc and also probably by copper and chlorine replacing zinc and sulfur respectively. This fluorescence is excited by a much stronger fluorescence around 350 nm, clearly in the UV range! This emission is caused by vacancies of zinc and sulfur acting as a "donor-acceptor" pair.
Measured with an Ocean Optics Flame spectrometer with backscattering/
reflection probe and a LED light source of 255 nm.