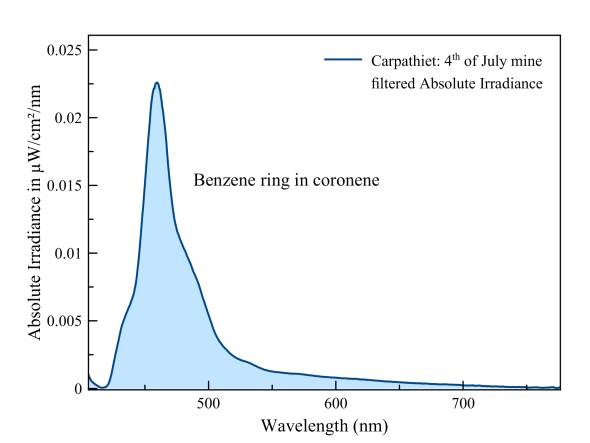
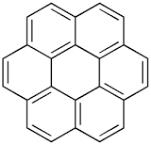
Carpathite (Karpathiet)
The fluorescence has two causes: 1) the aromatic component, the benzene ring and 2) enclosed aromatic polycyclic substances specific to the conversion of plants to coal. Two causes, two peaks... Move mouse arrow over graph to see the difference with whewellite.
Measured with: Ocean Optics Flame spectrometer and a LED light source of 360 nm with refection-backscattering probe. Measurement through a UV filter to prevent saturation of the excitation peak, which improves the dynamic range of the measurement.
The π-bonds of the benzene rings in the structure of coronene cause this fluorescence. Raw data of this spectrum is available for download
Mellite
Whewellite
The emission consists of two components:
A) the fluorescence of the π-bonds in the polycyclic aromatic hydrocarbons (PAHS) that typically occur in minerals in coal or brown coal conversions. This emission is somewhat redshifted due to conjugation of the double carbon bonds. This happens with the spectrum of mellite too but there is also the “added” emission of a benzene ring (on the blue side of the emission). Move mouse arrow over graph to see the difference.
B) the phosphorescence of the triplet-singlet transitions in those same PAHS.
Measured with: Ocean Optics Flame spectrometer and a LED light source of 255 nm with refection-backscattering probe. Measurement through a UV filter to prevent saturation of the excitation peak, which improves the dynamic range of the measurement.
Measured with: Ocean Optics Flame spectrometer and a LED light source of 255 nm with refection-backscattering probe. Measurement through a UV filter to prevent saturation of the excitation peak, which improves the dynamic range of the measurement.