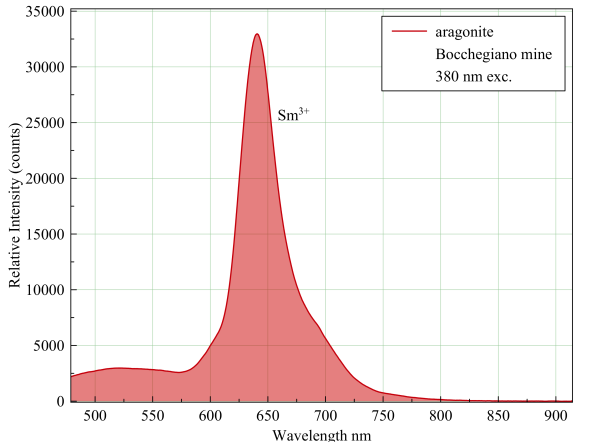
Measured with: Ocean Optics Flame spectrometer, backscattering/reflection probe and a 380 nm LED light source.
Workgroup Fluorescence
Aragonite, Bocchegiano mines
The narrow peak is indicative for electron transitions within the same electron shell. In most cases this means: lanthanide series elements. In this particular fluorescence it is samarium, replacing calcium, that lights up the specimen. Another clue is the fact that the fluorescence changes from deep red to light pink at shorter excitation wavelengths. Samarium, in its trivalent form, loves to be excited with energies that lie around 400 nm or slightly above. At shorter wavelengths it fluoresces only weak in these specimens because of the secundary fluorescence of humic acids. These organic molecules fluoresce in the 400 nm to 550 nm range, thereby activating the Sm3+. Without the humic acids, the specimen wouldn't fluoresce under SW at all. (Gorobets & Rogojine: Luminescent spectra of minerals, reference book)